879
Views & Citations10
Likes & Shares
Gene therapy is a very challenging field, especially with new emerging genetic disorders. Chitosan (CS) has been of interest in the world of gene therapy especially as researchers are gravitating towards non-viral vectors due to the problems caused by viral vectors. There has been a growing amount of effort to utilize chitosan and its derivatives in delivery of therapeutic agents, due to chitosan’s flexibility, biocompatibility, and biodegradability. Nevertheless, there are still issues regarding solubility, cellular uptake of cargos being transported in vitro or in vivo, increased cytotoxicity levels, as well as many other things that prevent chitosan from being an efficient drug delivery agent. This review will focus on the applications of chitosan and chitosan-based carriers such as tissue engineering, drug delivery, gene delivery, and wound healing.
Keywords: Biocompatibility, Biodegradability, Flexibility, Cytotoxicity levels, Tissue engineering
INTRODUCTION
Biological characteristics of CS and its derivatives
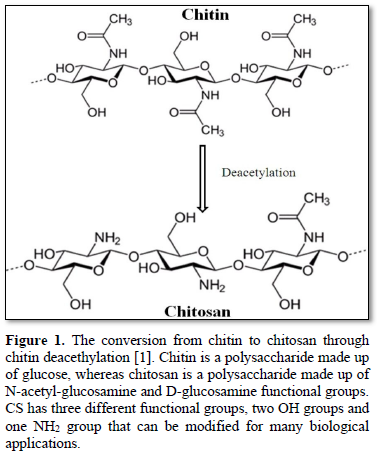
Since its discovery in mid-1800s, chitosan has been used in tissue regeneration, wound healing, cancer therapy, gene therapy, drug delivery, among many other things [1-7]. A table summarizing many recent applications of chitosan and its derivatives is included here (Table 1). Chitosan can be derived from chitin housed on the exoskeleton of insects and crustaceans, such as lobsters, crabs, and shrimp [1,2,7]. Conversion of chitin to CS is shown in Figure 1. The degree of acetylation in chitin is around 90%, while chitosan is a fully or partially N-deacetylated derivative with a typical degree of deacetylation of more than 65%. Chitosan is comprised of glucosamine and N-acetylated glucosamine units that are linked through b- (1-4) glycosidic linkages (Figure 1) [8]. The glucosamine of chitosan has primary amine groups with pKa of 6.5, which allows the biopolymer to only be soluble in acidic solution but not neutral or basic solution [5,9]. The activity of chitosan is influenced by Degree of Acetylation (DA) due to charge change upon deacetylation. It was reported that chitosan with 25% DA had a lower antibacterial activity than CS with 5% DA [1,10]. Low acetylation degree constituted for higher positive charges per molecule of chitosan, which is important for stability of the polyplex formed when the polymer complexes with a gene or a drug [5].
Modification of OH and NH2 functional groups on CS
Chemical modification of CS can be used to attain derivatives with preferred properties, and these modifications have proven to be safe for gene therapy usage. For instance, chitosan-DNA complexes integrated into THP-1 leukemia cells did not stimulate the release of pro-inflammatory cytokines [11]. Chitosan has two types of reactive groups that can be modified: 1) free amino groups on deacetylated units and 2) hydroxyl groups on acetylated or deacetylated units such as the -OH groups on C-3 and C-6 carbons (Figure 1) [1,2,12]. Chitosan's cationic property from the amino groups is a main advantage, especially in dealing with negative phospholipids or nucleic acids. These NH2 groups can be protonated to NH3+ in an acidic condition and as a result, these protonated NH3+ groups are able to form stable polymer complexes with anionic counterparts such as nucleic acids [13,14]. Along with these primary amino functional groups, chitosan’s OH groups offer many biological applications via their chemical alteration [1,15,16]. The N-alkylation reaction occurs on the C2-NH2 group because this group is a stronger nucleophile than the OH group, and thus O-alkylation, which could occur at C3-OH or C6-OH, is less likely to occur. N-alkylated chitosan derivatives have shown antibacterial and coagulation properties and have thus been used to prepare medical supplies such as medical gauze. This type of derivative has also served as a surfactant for water purification engineering as well.5 Although O-alkylation is less likely to occur, the hydroxyl group can be carboxylated by reacting with glyoxylic acid or chloroalkanoic acid. The carboxylation reaction, which increases the solubility of chitosan in water, occurs at the C6-OH group, allowing it to be dissolve in a pH greater than 7. Whereas carboxylation at the C3-OH group is more difficult due to steric hinderance [5,14].
Applications of chitosan
Chitosan has been incorporated in the production of bandages due to their anti-bacterial properties, as well as enzyme immobilization on solid surfaces and surface coating of enzymes, which allows for reusability, thus promoting cost-effectiveness [17,18]. Other applications of chitosan include food preservation, as shown in a study that it was used for the promotion of crop growth such as radishes. It has also been used to minimize water loss in field crops, enhance enzyme activity in peanuts, and to prolong the storage of fruits by stimulating cellular defense compounds [1]. However, there have been rare incidences by which chitosan produced undesirable characteristics. For example, a study that was investigating the effects of chitosan-based gene therapy on amniotic fluid (in vitro) reported that while CS protected the plasmid DNA from degradation, it also formed aggregates in the amniotic fluid, thus meaning that CS can pose a threat during fetal development [19].
Uses of chitosan
Chitosan has been incorporated in the production of bandages due to their anti-bacterial properties, as well as enzyme immobilization on solid surfaces and surface coating of enzymes, which allows for reusability thus promoting cost-effectiveness [17,18]. Other applications of chitosan include food preservation, as shown in a study that it was used for the promotion of crop growth such as radishes. It has also been used to minimize water loss in field crops, enhance enzyme activity in peanuts, and to prolong the storage of fruits by stimulating cellular defense compounds [1]. In its natural state, chitosan has some shortcomings such as low transfection efficiency and low solubility in physiological pH. The polymer can be chemically modified to attain derivatives with preferred properties, and this modification has been proven to be safe for gene therapy usage. When conjugated to DNA and integrated into THP-1 leukemia cells, the chitosan-DNA complex did not stimulate the release of pro-inflammatory cytokines. In a study investigating the effects of chitosan-based gene therapy on amniotic fluid (in vitro), it protected the plasmid DNA from degradation; although chitosan was aggregated in the amniotic fluid [19]. As these CS nanoparticles are internalized via endocytosis, they can deliver drugs into cells without endangering these biologically active cargos [17, 20-22]. N/P ratio is the ratio of positively charged amino group on chitosan to negatively charged phosphate group on nucleic acids [2]. High N/P ratio leads to strongly formed polyplex through electrostatic interaction, and if the bond between the DNA and the polymer is too strong, this can prevent the nucleic acid from being released once it arrives at its site of action [2,23].
Gene delivery using chitosan and its derivatives
Gene therapy is the delivery of genes into specific cells for therapeutic benefit and can offer possible lasting treatment for cancer. A challenge for gene therapy is to design a carrier that is effective in protecting the gene of interest from nucleases as well as efficient transfer to targeted cells. Viruses are the most common vectors for gene therapy, although gene therapy using viral vectors is associated with immunogenicity as well as rare cases of disease [24]. However, Due to its versatility, biodegradability, and safety, chitosan has recently been gaining interest as a potential non-viral vector in the field of gene therapy. Additionally, stability in a biological environment is a quality all ideal DNA/RNA delivery vectors should have. How does chitosan protect genetic material from being degraded by nucleases? Through the CS-DNA polyplex formation, the positively charged amine groups on chitosan interact with the negatively charged groups on DNA/RNA in order to form a stable CS-DNA/RNA polymer complex [2,25,26].
In 1995, the first ever non-viral gene delivery experiment using chitosan was performed using N, N, N-trimethyl chitosan polymers (TMC) [27]. TMC is an ammonium quaternized chitosan derivative, first synthesized in 1986, and has been proven to have mucoadhesive properties. This derivative is synthesized by addition of trimethyl group to chitosan’s NH2 group. The process of synthesizing TMC involves trimethylation of the primary amines of chitosan in alkaline solution. Kean et al. measured the transfection efficiency of pGL3 luciferase plasmid-DNA using TMC and TMOs (trimethylated chitosan oligosaccharides) at differing degrees of trimethylation (DTM) in MCF-7 breast cancer and COS-7 monkey kidney fibroblast-like cell lines. The authors found that the transfection was cell dependent. Moreover, TMOs had the higher transfection efficiencies in both cell lines than most of the TMC derivatives. However, in the MCF-7 cell line, 93% TMC which was the TMC derivative with the greatest degree of trimethylation (93% DTM) had the greatest transfection efficiency.
Nevertheless, TMC showed higher cytotoxicity compared to the unmodified chitosan. Usually, caveats to TMC are related to its molecular weight (MW); a MW of 400 kDa displayed high cytotoxicity, while 5 and 25 kDa TMC exhibited little to no toxicity [1,45,46]. Research has shown that the cytotoxicity is due to the positive charge of TMC which might be interacting with the negatively charge cell membrane which could lead to cell membrane damage [1,28,29].
Another category of chitosan derivative used for gene delivery are thiolated chitosans. Due to their cell permeability and mucoadhesive properties, these types of derivatives show enhanced polymer-DNA complex stability and excellent gene delivery both in vivo and in vitro [30]. During reducing conditions, breakage of the disulfide bonds lead to dissociation of the DNA being delivered, whereas during oxidation, the disulfide bond formation in the thiolated chitosan is favored, which leads to tight binding of DNA and a stable solid polyplex. Thiolated chitosan derivatives were discovered in the early 2000s, especially novel derivative chitosan-TBA in which chitosan is conjugated to 4-thiobutylamidine. A group performed pDNA transfection in the Caco-2 cell line and found that when treated with a nuclease, the pDNA was protected by chitosan-TBA, and the chitosan-TBA-pDNA complex displayed stability. In vitro hemolysis experiments were performed using chitosan-TBA to evaluate its safety on red blood cells. The derivative displayed a low hemolytic effect on the red blood cells which might have been attributed to the change of the primary amine moieties into secondary amine groups after thiol modification with TBA [1,30].
There are very few reports on the cellular uptake mechanisms of CS-DNA/RNA polyplexes. Hashimoto et al. synthesized mannosylated chitosan (Man-C) with 5% and 21% degree of substitutions for use as gene carriers into mouse peritoneal macrophages and COS7 cells, in order to understand the cellular uptake of CS-pDNA polyplex. The authors discovered that transfection with both 5% and 21% Man-C were better than unmodified chitosan in the macrophages, but in the COS7 cells, the carrier efficiency was greater with the 5% Man-C than 21% Man-C derivative [26,31]. The Man-C derivative with 21% substitution might have been causing extracellular damage due to 21% Man-C being more hydrophilic than the unmodified and 5% chitosans.
Additionally, a factor that affects gene therapy is the CS-nucleic acid N/P ratio. N/P ratio is the ratio of positively charged amino group on chitosan to negatively charged phosphate groups on nucleic acids [2]. N/P ratio influences the stability of the polyplex formation, the transfection efficiency, and polymer-cell interactions [2]. A study compared transfection efficiencies of 6-amino-6-deoxy-chitosan (6ACT) at N/P ratio of 2.5 and chitosan-DNA at N/P ratio of 5 and found that the 6ACT derivative was the better gene carrier. 6ACT-DNA and CS-DNA complexes were also compared to polyethyleneimine (PEI) which had a higher N/P ratio, and the results showed that PEI had better transfection efficiencies than both complexes [32]. High N/P ratio can lead to strongly formed polyplex through electrostatic interactions. However, if the bond between the DNA and the polymer is too strong, this can prevent the nucleic acid from being released once it arrives at its site of action, thus hindering the release of the gene from the polymer complex [2,23]. Additionally, an extremely low N/P ratio can cause formation of aggregates which affect cell internalization, thus resulting in poor transfection [26].
Drug delivery using CS and its derivatives
Although chitosan has anticoagulant properties and was involved in wound healing, it wasn’t until the late 1990s that the chitosan was used as a drug delivery carrier. This is mainly due to chitosan’s solubility issues, which prevents it from delivering the drug to biological systems [22]. Moreover, due to unmodified chitosan’s pKa of 6.5 it is not a stable drug carrier. Drug delivery agents must be stable at physiological pH. In order to overcome this problem, there have been many attempts to modify chitosan by derivatizing the OH or NH2 groups on the polymer. Common types of modifications to make chitosan more biocompatible include quaternization, sulfonation, carboxymethylation, N- and O-hydroxyalkylation [13]. Examples of hydrophilic modifications to chitosan listed in this review include quaternization using ammonium groups and N-modification with the succinyl group. One study demonstrated the usage of N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride (HTCC) as a drug delivery agent for ribavirin, and the results showed an initial burst release of the drug at increasing degree of substitution (Table 1) [3,5,15,16]. As modified CS derivatives are internalized via endocytosis, they are able to deliver therapeutic drugs into cells without endangering these biologically active cargos [17,20-22].
Modified chitosans such as TMC show promise results in the intranasal administration of certain drugs and proteins, due to their cationic properties that allow for complexation with the drug of interest. Intranasal administration allows for direct access to the brain, which has many advantages such as rapid onset of action and fewer adverse effects [33]. Turabee et al. developed a hydrogel made from pluronic F127 (PF127) in order to treat the malignant glioblastoma cell line U87MG. They discovered that after addition of TMC to the PF127 hydrogel, delivery and release of the anticancer drug docetaxel (DTX) was much better compared DTX alone or DTX encapsulated with PF127, in vitro. The authors using a murine model were able to show that PF127-TMC hydrogel was capable of tumor suppression, in vivo, with the delivery of DTX [13,34].
Moreover, in 1994, a patent was requested to use N-succinyl-chitosan or Suc-Chi (originally developed in the late 1990s for wound dressing) as a treatment for arthritis. Suc-Chi exhibits low toxicity, is biocompatible, and can be retained for a long period of time in the body. It has been used as a drug carrier and when conjugated with the chemotherapeutic drug mitomycin C, it presented antitumor activity against many tumors (Table 1) [1,35]. Furthermore, chitosan loaded with dopamine was proven to minimize cytotoxicity and used to facilitate transport the dopamine across the blood brain barrier for Parkinson’s disease [26,36,37].
However, certain types of cargo such as hydrophobic drugs are problematic due to their hydrophobic nature. In addition, due to chitosan’s insolubility in hydrophobic solvents, its ability to transport hydrophobic drugs is restricted. In order to resolve this limitation, researchers began chemically modifying chitosan with hydrophobic groups such as pyridine, among many other groups, to improve the ‘encapsulation efficiency’ of the hydrophobic cargo [13,38]. Examples of hydrophobic modifications include palmitoyl units and the carboxymethyl group. When chitosan was substituted with palmitoyl units (degree of substitution 40-50%), its best drug release characteristics were displayed, which proved that palmitoyl chitosan could be used in subdermal and oral drug delivery applications (Table 1) [39-41]. N, O-carboxymethyl chitosan (N, O-CMC) nanoparticles, in a study performed in 2010, demonstrated that a derivative of chitosan could be used to carry hydrophobic chemotherapeutic drugs such curcumin (Table 1) [1,42,43]. Nevertheless, hydrophobic modification of chitosan polymer has low reproducibility, proving that chitosan is not as successful in delivering hydrophobic drugs [44].
Chitosan-coated materials and complexes
Chitosan and its derivatives have also been used as encapsulating agents for proteins such as bovine serum albumin, hemoglobin, and dextran due to their high affinity for the cell membrane [40]. In addition, chitosan was used to coat PLGA (poly (lactic-co-glycolic acid)) microparticles that contain tetanus toxoid, improving the stability of the drug and PLGA by preventing degradation by lysozyme. Furthermore, it was found that the coating enhances the nasal transport of the drug due to chitosan’s mucosal adhesion or mucoadhesive properties.
A study demonstrated that conjugation of chitosan to poly (acrylic acid) (PAA), an anionic polyelectrolyte, was useful for delivery of gastric antibiotic drugs due to the chitosan-poly (acrylic acid (Ch-PAA) derivative being stable under acidic conditions [40].
It is widely accepted that modified chitosans have chemical properties superior to unmodified chitosan, yet unmodified chitosan has many favorable characteristics such as biocompatibility, low cytotoxicity, biodegradability, and stability when forming complexes, which are desirable qualities for an effective gene delivery agent.2 Unmodified chitosan was used for the removal of organic matter such as algae, proved to be more efficient than the following coagulating inorganic compounds (Al2(SO4)3, KAl(SO4)2, Ca(OH)2), by removing about 95% of algae from algae-containing waters.1 Regardless of these characteristics, unmodified chitosan has certain constraints, a main one being solubility. Chitosan is insoluble in physiological pH. Thus, it is necessary for modifications that make it easier to incorporate the biopolymer into cells.
Tissue engineering applications using CS derivatives
Chitosan’s ability to be refined into porous material is an excellent characteristic that is useful for tissue engineering applications because it can be made into scaffold grafts for tissue engineering [45-47]. These scaffold grafts stimulate the regeneration of certain types of tissues such as bone tissue, among many others. Tissue engineering using chitosan-based matrices in transplantation procedures of bovine adrenal chromaffin cells was discovered in 1998 (Table 1) [48-50]. Since then chitosan and its derivatives have been used in tissue regeneration due to their low to non-existent tissue toxicity, biodegradability, as well as peritoneal adhesion prevention.21 A previous study reported that modification of chitosan with multiple proteins such as collagen, gelatin, and albumin enhanced its biocompatibility. The results demonstrated that a matrix made up of collagen modified chitosan attached more readily to the cells than the other proteins (Table 1) [48]. Additionally, chitosan’s hydrophilic surface promotes cell proliferation and adhesion much better than several synthetic polymers. Chupa et al. reported the use of both heparin-CS and dextran sulfate (DS)-chitosan complexes to stimulate cell proliferation and tissue regeneration of human endothelial cells and smooth muscle cells, in vivo [45,48,51]. Thus, proving the potential of CS to be used in scaffolds that enhance and promote cell and eventually tissue regeneration.
Chitosan-coated materials and complexes
Unmodified chitosan was used for the removal of organic matter such as algae, proved to be more efficient than the following coagulating inorganic compounds (Al2(SO4)3, KAl(SO4)2, Ca(OH)2), by removing about 95% of algae from algae-containing waters [1]. Chitosan and its derivatives have also been used as encapsulating agents for proteins such as bovine serum albumin, hemoglobin, and dextran due to their high affinity for the cell membrane [40]. In addition, chitosan was used to coat PLGA (poly (lactic-co-glycolic acid)) microparticles that contain tetanus toxoid, improving the stability of the drug and PLGA by preventing degradation by lysozyme. Furthermore, it was found that the coating enhances the nasal transport of the drug due to chitosan’s mucosal adhesion or mucoadhesive properties. A study showed that a chitosan conjugation to poly (acrylic acid) (PAA), an anionic polyelectrolyte, is useful for delivery of gastric antibiotic drugs because chitosan-poly (acrylic acid (Ch-PAA) complex is stable under acidic conditions [40]. Though it is widely accepted that modified chitosans have chemical properties superior to unmodified chitosan, yet unmodified chitosan has many favorable characteristics such as biocompatibility, low cytotoxicity, biodegradability, stability when forming complexes, which are desirable qualities for effective gene delivery agent [2]. Regardless of these characteristics, unmodified chitosan has certain constraints, a main one being solubility. Chitosan is insoluble in physiological pH, thus leading to modifications that make it easier to incorporate the biopolymer into cells. In this review, the current progress using CS derivatives is discussed and summarized as well as limitations of CS and its derivative.
Tissue engineering applications using CS derivatives
Chitosan’s ability to be refined into porous material is an excellent characteristic that useful for tissue engineering applications because it that can be made into a scaffold grafts for tissue engineering [45-47]. Tissue engineering using chitosan-based matrices in transplantation procedures of bovine adrenal chromaffin cells was discovered in 1998 (Table 1) [48-50]. In a previous study, chitosan was modified using multiple proteins such as collagen, gelatin, and albumin to enhance its biocompatibility. The results demonstrated that a matrix made up of collagen modified chitosan attached more readily to the cells than the other proteins (Table 1) [48]. Chitosan and its derivatives have been used in tissue regeneration due to their low to non-existent tissue toxicity and also properties that allow for degradation by lysozyme, as well as peritoneal adhesion prevention [21]. Additionally, the hydrophilic surface of chitosan promotes cell proliferation and adhesion much better than several synthetic polymers. A study reported that dextran sulfate (DS)-chitosan complexes stimulated cell proliferation and tissue regeneration of human endothelial cells and smooth muscle cells, in vitro [45,48,51].
Wound healing and anticoagulant properties of CS and its derivatives
Wound healing using chitosan was first discovered in late 1980s-early 1990s [13,52]. Studies have shown that chitosan improves skin wound healing and facilitates re-epithelization. There have been numerous articles investigating chitosan and its derivatives’ influence on peritoneal adhesion formation. These studies demonstrated that as the degree of acetylation on chitosan biofilms increased, cell proliferation and adhesion decreased [53]. Chitosan also reduces inflammation at the wound site, and promotes dermal regeneration. Additionally, the biopolymer has the capability to accelerate wound healing because it stimulates macrophages and attracts neutrophils [1,54,55].
Studies have shown that derivatives of CS substituted with sulfonate groups have high anticoagulant activity due to their similarity with heparin, a common anticoagulant medication. These sulfonated derivatives have no side-effects and are less expensive compared to heparin and have in fact have replaced heparin in many pharmaceutical and clinical settings [1]. Examples of such derivatives are N-Sulfofurfuryl chitosan, O-sulfonated chitosan, and N, O-sulfonated chitosan. Amphoteric water-soluble chitosan derivatives such as N-sulfofurfuryl chitosan and N-benzyl sulfonate chitosan, possess non-thrombogenic properties and can be used for blood-coagulating applications. They have also been used in wastewater applications [1]. N, O-sulfonated chitosan also showed strong anticoagulant activities by inhibiting thrombin activity, as did O-sulfonated chitosan, which in addition was reported to have excellent inflammation inhibition activities (Table 1) [1]. These sulfonated chitosan derivatives are also used to remove organic pollutants and heavy metals, such as Cd2+ , Zn2+ , Ni2+ , Pb2+ , Cu2+ , Fe3+ , and Cr3+ from industrial sewages and proved to remove these pollutants and heavy metals much better than unmodified chitosan could (Table 1) [1,56].
Antioxidant and antimicrobial properties of CS and CS derivatives
In 1992 chitosan was discovered to have antioxidant and antimicrobial properties in Japan, the authors were trying to investigate macrophage activation and antimicrobial activity of chitosan and chitin [57]. Chitosan as well as derivatives of chitosan, especially low molecular weight chitosan (LMWC), has been proven to display strong scavenging activity towards superoxides and H2O2, and 2,2-diphenyl-1-picrylhydrazyl, (DPPH) radicals [1,57]. A study measuring the antioxidant activity of unmodified chitosan and two derivatives, double N-quarternized chitosan (DQCS) and single N-quarternized chitosan (QCS), demonstrated that DQCS had the best scavenging ability compared to both unmodified CS and QCS in the presence of DPPH, hydroxyl radicals and superoxide radicals.4
Another article by Sinha et al. [58] investigated the antimicrobial activities of chitosan crosslinked with fatty acids (lauric, stearic, and capric saturated fatty acids) as well as drug delivery of ciporoflaxacin against S. aureus and E. coli. They discovered that the chitosan-lauric acid derivative and the chitosan-lauric acid-ciporoflaxacin complex had inhibitory effects against the two microbes. When unmodified chitosan was complexed with lipopolysaccharide (LPS), it was reported to have inhibited cytokine production in macrophages (RAW 264.7) [1,13,58]. The CS-LPS complex led to more enhanced phagocytic activity of the macrophages than when the macrophages were stimulated with LPS alone. Another way chitosan demonstrates its antioxidant properties is that it protects from hypertrophy of adrenal glands (induced by LPS), prevents from shrinkage of the thymus, changes of hormones, glycolysis and glycogenolysis activation, and lipid peroxidation in liver cells [1].
Limitations of Chitosan and its future use
The derivatives aforementioned have demonstrated the various applications and versatility of chitosan in many different fields. However, modifications on chitosan such as thiol addition, quarternization on the amino groups, addition of hydrophobic groups, addition of sulfono group, etc. can sometimes prevent delivery of certain nucleic acids and drugs. Although in most cases have increased the versatility of chitosan. For instance, hydrophobic modification of CS results in slow release. Limitations of chitosan that impact drug and gene delivery are due to pKa, degree of acetylation, N/P ratio, and MW. High MW chitosan can lead to unstable CS-DNA polyplex formation as well as problems with cellular uptake and release of the nucleic acid into the cytoplasm [1,25,59,60]. Although, DA and MW can become advantageous in chitosan becoming a vector for drug and gene delivery, e.g. through modification, other limitations include an increase in cytotoxicity as well as a reduction in gene binding capacity when the biopolymer has been modified with a high degree of substitution [59]. Whereas N/P ratio can variable with CS derivatives. Although N/P ratio between 1-5 usually allows for stable polyplex formation, with and N/P ratio of 2.5 usually being the optimal ratio for most CS derivatives. In contrast, N/P ratio make it polyplex formation difficult and lead to aggregate formation.
However, any of these derivatives have proven to have good characteristics such as high gene delivery capacity, protection of cargo from lysozyme degradation, low cytotoxicity and solubility. Nevertheless, cytotoxicity could be caused by steric effects in N-substituted (quaternization of the amino groups on chitosan) derivatives such as TMC which can be seen in many of the chitosan derivatives.28, 29 This could serve as a precaution to researchers as they investigate certain degree of substitutions that would make chitosan a more efficient gene delivery vector. In order for these derivatives to be efficient gene carriers, they must demonstrate excellence in these characteristics. With this information, research should be geared towards using chitosan and its derivatives in treating of genetic and autoimmune disorders, as more awareness about these diseases is being brought to light.
Moreover, factors such as degree of deacetylation, ionic strength of the solution, and the molecular weight influence the solubility of chitosan and its ability to be an efficient delivery agent [16,36]. Another limitation of chitosan includes the inability to release therapeutic cargo intracellularly after endocytosis when complexed with DNA, thus leading to a less efficient delivery of DNA, in vitro [23,61,62]. Endo-lysosomal release of these genetic materials into the cell is just as important as internalization in the cell [6,61,63,64]. As these CS nanoparticles are internalized via endocytosis, they can deliver drugs into the cytoplasm without endangering these biologically active cargos. Although chitosan has been used in drug delivery applications as well as its effects on cell viability using many derivatives, there are very few gene therapy-based studies using chitosan modified with ammonium or phosphonium salts such as TEPB-CS and TEAB-CS. Therefore, the aim of the present work is to determine if these chitosan derivatives can be used as efficient gene delivery vectors [8,60]. Future chitosan research could be geared towards better understanding of intercellular processes involving chitosan as there currently few in-depth studies about this topic especially because chitosan and its derivatives can be used in therapy for many different diseases [68].
1. Tait JM, Mackay RG (2012) Handbook of Chitosan Research and Applications. Nova Science Publishers, Inc: Hauppauge, N.Y., 2012.
2. Cao Y, Tan YF, Wong YS, Liew MWJ, Venkatraman S (2019), Recent advances in chitosan-based carriers for gene delivery. Marine Drugs 17(6): 381.
3. Li SD, Li PW, Yang ZM, Peng, Z, Quan WY (2014) Synthesis and characterization of chitosan quaternary ammonium salt and its application as drug carrier for ribavirin. Drug Delivery 21(7): 548-552.
4. Luan F, Wei L, Zhang J, Tan W, Chen Y, et al. (2018) Preparation and characterization of quaternized chitosan derivatives and assessment of their antioxidant activity. Molecules 23(3): 516.
5. Wang W, Meng Q, Li Q, Liu, J, Zhou M, et al. (2020) Chitosan derivatives and their application in biomedicine. Int J Mol Sci 2020(2): 487.
6. Rizeq BR, Younes NN, Rasool K, Nasrallah GK (2019) Synthesis, bioapplications, and toxicity evaluation of chitosan-based nanoparticles. Int J Mol Sci 20(22): 5776.
7. Mumper RJ, Wang J, Claspell JM, Rolland AP (1995) Novel polymeric condensing carriers for gene delivery. Proc Int Symp Controlled Release Bioact Mater 22: 178.
8. Lin G, Zhang H, Huang L (2015) Smart polymeric nanoparticles for cancer gene delivery. Mol Pharm 12(2): 314-321.
9. Qi L, Xu Z, Jiang X, Li Y, Wang M (2005) Cytotoxic activities of chitosan nanoparticles and copper-loaded nanoparticles. Bioorg Med Chem Lett 15(5): 1397-1399.
10. Wenqian W, Qiuyu M, Qi L, Jinbao L, Mo Z (2020) Chitosan derivatives and their application in biomedicine. Int J Mol Sci 21(2): 1-26.
11.Chellat F (2004) Metalloproteinase and cytokine production by THP-1 macrophages following exposure to chitosan-DNA nanoparticles. Biomaterials 2004: 26.
12. Mao HQ, Roy K, Troung-Le VL, Janes KA, Lin KY, et al. (2001) Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release 70: 399.
13. Kravanja G, Primožič M, Knez Ž, Leitgeb M, Chen JP (2019) Chitosan-based (nano)materials for novel biomedical applications. Molecules 24(10): 1960.
14. Zhang J, Xia W, Liu P, Cheng Q, Tahirou T, et al. (2010) Chitosan modification and pharmaceutical/biomedical applications. Marine Drugs 8(7): 1962-1987.
15. Layek B, Singh J (2017) Chitosan for DNA and gene therapy, pp: 209-244.
16. Layek B, Lipp L, Singh J (2015) Cell penetrating peptide conjugated chitosan for enhanced delivery of nucleic acid. Int J Mol Sci 16(12).
17. Li J, Cai C, Li J, Sun T, Wang L (2018) Chitosan-based nanomaterials for drug delivery. Molecules 23(10): 2661.
18. Periayah MH, Halim AS, Saad AZ.M (2016) Chitosan: A promising marine polysaccharide for biomedical research. Pharmacogn Rev 10(19): 39-42.
19. Yang PT, Hoang L, Jia WW, Skarsgard ED (2011) In utero gene delivery using chitosan-DNA nanoparticles in mice. J Surg Res 171(2): 691-699.
20. Zubareva A, Svirshchevskaya E (2016) Interactions of chitosan and its derivatives with cells (review). Appl Biochem Microbiol 52(5): 465-470.
21. Bowman K, Leong KW (2006) Chitosan nanoparticles for oral drug and gene delivery. Int J Nanomed 1 (2): 117-128.
22. Garg U, Chauhan S, Nagaich U, Jain N (2019) Current advances in chitosan nanoparticles-based drug delivery and targeting. Adv Pharm Bull 9(2): 195-204.
23. Dubashynskaya N, Poshina D, Raik S, Urtti A, Skorik YA (2020) Polysaccharides in ocular drug delivery. Pharmaceutics 12(1): 22.
24. Layek B, Haldar MK, Sharma G, Lipp L, Mallik S, et al. (2014) Hexanoic acid and polyethylene glycol double grafted amphiphilic chitosan for enhanced gene delivery: Influence of hydrophobic and hydrophilic substitution degree. Mol Pharma 11(3): 982-994.
25. Borchard G (2001) Chitosans for gene delivery. Adv Drug Delivery Rev 52: 145.
26. Xu Q, Wang CH, Pack DW (2010) Polymeric carriers for gene delivery: Chitosan and poly(amidoamine) dendrimers. Curr Pharm Des 16(21): 2350-2368.
27. Özgümüş S, Gök M, Pabuccuoğlu S (2016) Chitosan: Gene Delivery, pp: 15.
28. Kean T, Roth S, Thanou M (2005) Trimethylated chitosans as non-viral gene delivery vectors: Cytotoxicity and transfection efficiency. J Control Release 103: 643-653.
29. Germershaus O, Mao S, Sitterberg J, Bakowsky U, Kissel T (2008) Gene delivery using chitosan, trimethyl chitosan or polyethylenglycol-graft-trimethyl chitosan block copolymers: Establishment of structure-activity relationships in vitro. J Control Release 125: 145-154.
30. Inamdar N, Mourya VK (2013) Thiolated Chitosan: Preparation, properties and applications, pp: 121-150.
31. Hashimoto M, Morimoto M, Saimoto H, Shigemasa Y, Yanagie, H (2006) Gene transfer by DNA/mannosylated chitosan complexes into mouse peritoneal macrophages. Biotechnol Lett 28(11): 815-821.
32. Satoh T, Kakimoto S, Kano H, Nakatani M, Shinkai, S, et al. (2007) In vitro gene delivery to HepG2 cells using galactosylated 6-amino-6-deoxychitosan as a DNA carrier. Carbohyd Res 342: 1427-1433.
33. Ojeda-Hernández DD, Canales-Aguirre AA, Matias-Guiu J, Gomez-Pinedo U, Mateos-Díaz JC (2020) Potential of chitosan and its derivatives for biomedical applications in the central nervous system. Front Bioengg Biotech 8: 389.
34. Turabee MH, Jeong T, Ramalingam P, Kang J, Ko YT (2018) N, N, N-trimethyl chitosan embedded in situ Pluronic F127 hydrogel for the treatment of brain tumor. Carbohyd Polym 203.
35. Babu A, Ramesh R (2017) Multifaceted applications of chitosan in cancer drug delivery and therapy. Marine Drugs 15 (4): 96.
36. Singh AP, Biswas A, Shukla A, Maiti P (2019) Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Sign Transduct Target Ther 4(1): 33.
37. Rai R, Alwani S, Badea I (2019) Polymeric nanoparticles in gene therapy: New avenues of design and optimization for delivery applications. Polymers 11(4): 745.
38. Nishimura S, Kohgo O, Kurita K, Kuzuhara H (1991) Chemospecific manipulations of a rigid polysaccharide: Syntheses of novel chitosan derivatives with excellent solubility in common organic solvents by regioselective chemical modifications. Macromolecules 24(17): 4745-4748.
39. Omid N, Babanejad N, Amini H, Amini M, Tehrani RM, et al. (2014) Preparation and characterization of novel derivatives of chitosan and trimethyl chitosan conjugated with dipeptides and vitamin B12 as candidates for oral delivery of insulin. J Polym Res 21(8): 1-16.
40. Prabaharan M, Mano JF (2005) Chitosan-based particles as controlled drug delivery systems. Drug Deliv 12(1): 41-57.
41. Suksamran T, Kowapradit J, Ngawhirunpat T, Rojanarata T, Sajomsang W (2012) Oral Methylated N-Aryl chitosan derivatives for inducing immune responses to ovalbumin. Trop J Pharm Res 11(6): 899-908.
42. Anitha A, Maya S, Deepa N, Chennazhi KP, Nair SV (2012) Curcumin-Loaded N,0-Carboxymethyl chitosan nanoparticles for cancer drug delivery. J Biomater Sci 23(11): 1381-1400.
43. Philippova OE, Volkov EV, Sitnikova NL, Khokhlov AR (2001) Two types of hydrophobic aggregates in aqueous solutions of chitosan and Its hydrophobic derivative. Biomacromolecules 2: 483.
44. Ways TM, Lau WM, Khutoryanskiy VV (2018) Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 10(3): 267.
45. Hutmacher D, Goh J, Teoh H (2001) An introduction to biodegradable materials for tissue engineering applications. Ann Acad Med 30: 183-191.
46. Rodríguez-Vázquez M, Vega-Ruiz B, Ramos-Zúñiga R, Saldaña-Koppel D, Quinones Olvera L (2015) Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res Int.
47. Levengood SL, Zhang M (2014) Chitosan-based scaffolds for bone tissue engineering. J Mater Chem B 2(21): 3161-3184.
48. Elçin E, Elçin M, Pappas G (1998) Neural tissue engineering: Adrenal chromaffin cell attachment and viability on chitosan scaffolds. Neurol Res 20(7): 648-654.
49. Hu FQ, Chen WW, Zhao MD, Yuan H, Du YZ (2013) Effective antitumor gene therapy delivered by polyethylenimine-conjugated stearic acid-g-chitosan oligosaccharide micelles. Gene Ther 20(6): 597-606.
50. Raftery R, O'Brien FJ, Cryan SA (2013) Chitosan for gene delivery and orthopedic tissue engineering applications. Molecules 18(5): 5611-5647.
51. Chupa J, Foster A, Sumner S, Madihally S, Matthew H (2000) Vascular cell responses to polysaccharide materials: In vitro and in vivo evaluations. Biomaterials 21: 2315-2322.
52. Matica MA, Aachmann FL, Tøndervik A, Sletta H, Ostafe V (2019) Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action. Int J Mol Sci 20(23): 5889.
53. Lim S, Song D, Cho K, Oh S, Lee-Yoon D (2007) Cell adhesion and degradation behaviors of acetylated chitosan films.
54. Dai T, Tanaka M, Huang YY, Hamblin, MR (2011) Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Exp Rev Anti-infect Ther 9(7): 857-879.
55. Ahmed S, Ikram S (2016) Chitosan based scaffolds and their applications in wound healing. Acheiv Life Sci 10(1): 27-37.
56. Weltrowski M, Martel B, Morcellet M (1996) Chitosan N-benzyl sulfonate derivatives as sorbents for removal of metal ions in an acidic medium. J Appl Polym Sci 59(4): 647-654.
57. Finch CA (1993) Advances in chitin and chitosan. Edited by C. J. Brine, P. A. Sandford and J. P. Zikakis. Elsevier Science Publishers, London, 1992. pp. xxii + 685, price £135.00. ISBN 1‐85166‐899‐3. Polym Int 31(4): 404-404.
58. Sinha N, Singh BK, Dutta PK (2016) Preparation and characterization of chitosan-lauric acid derivative: Antibacterial activity and drug delivery study. J Polym Mater 33(3): 479-489.
59. Kritchenkov AS, Andranovitš S, Skorik YA (2017) Chitosan and its derivatives: vectors in gene therapy. Russ Chem Rev 86(3): 231-239.
60. Sahariah P, Másson M (2017) Antimicrobial chitosan and chitosan derivatives: A review of the structure–activity relationship. Biomacromolecules 18(11): 3846-3868.
61. Rajoka RMS, Zhao L, Mehwish HM, Wu Y, Mahmood S (2019) Chitosan and its derivatives: Synthesis, biotechnological applications and future challenges. Appl Microbiol Biotech 103(4): 1557-1571.
62. Rajoka MSR, Zhao L, Mehwish HM, Wu Y, Mahmood S (2019) Chitosan and its derivatives: Synthesis, biotechnological applications and future challenges. Appl Microbiol Biotech 103(4): 1557-1571.
63. Zhao QQ, Chen JL, Lv TF, He CX, Tang GP, et al. (2009) N/P ratio significantly influences the transfection efficiency and cytotoxicity of a polyethylenimine/chitosan/dna complex. Biol Pharm Bull 32: 706-710.
64. Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS (2001) Chitosan as a novel nasal delivery system for vaccines. Adv Drug Delivery Rev 51: 81.
65. Park SB, You J, Park HY, Haam S, Kim W (2001) A novel pH-sensitive membrane from chitosan — TEOS IPN; preparation and its drug permeation characteristics. Biomater 22: 323-330.
66. Schuerer N, Stein E, Inic-Kanada A, Ghasemian E, Stojanovic M, et al. (2018) Effects of chitosan and chitosan N-acetylcysteine solutions on conjunctival epithelial cells. J EuCornea 1(1): 12-18.
67. Fangkangwanwong J, Akashi M, Kida T, Chirachanchai S (2006) Chitosan‐Hydroxybenzotriazole Aqueous solution: A novel water‐based system for chitosan functionalization. Macromol Rapid Commun 27: 1039-1046.
68. Kazemi S, Mohammadi Z, Amini M, Yousefi M, Tarighi P, et al. (2019) Thiolated chitosan-lauric acid as a new chitosan derivative: Synthesis, characterization and cytotoxicity. Int J Biol Macromol 2019: 136.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)